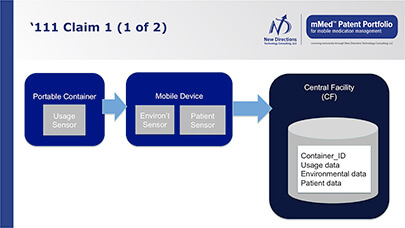
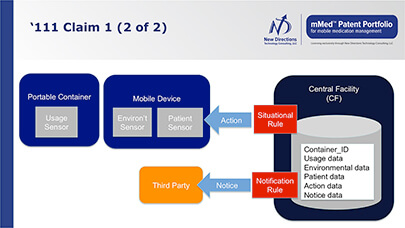
Benefits of Products and Systems
that Incorporate mMed Patent Claims
Systems based on the mMed patent portfolio offer numerous benefits to all healthcare stakeholders.
For Patent Acquirers and Licensees (Pharma or Others)
Benefits for purchasing or licensing patents include: (i) competitive barrier against others providing similar/same service, and (ii) ability to control who competes in your market via licensing. Benefits accrue to patent owners and licensors.
Pharma is being asked to demonstrate product value, evidence-based outcomes, and improvement in quality of life. With mobility being crucial to quality of life, there is a strong rationale for a Pharma emphasis on mHealth. Service beyond the script and mHealth/medication telemanagement are now key parts of pharmaceutical companies' strategies to gain and retain patients. This is ever more important in a healthcare environment in which patients, practitioners and others, assess the value of pharma products.
mHealth/medication telemanagement can provide real-time information to permit Pharma to manage the medications they provide. Such information can be of significant predictive value, as well.
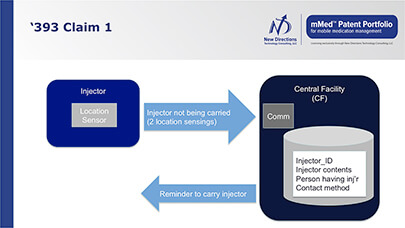
This can be particularly important when it comes to often-expensive specialty pharma products, including those used to treat such complex diseases as multiple sclerosis, rheumatoid arthritis, hepatitis C and cancer. Some are fragile and administered parenterally (to withstand first-pass metabolism or otherwise to enhance bioavailability), and injectable products pose special compliance and disposal issues.
The specialty pharma market is growing far faster than the overall market for pharmaceutical companies. More products are being compounded or engineered for specific patients or patient groups. Pharma has invested billions in the specialty-drug market primarily through the purchase, development and marketing of biotech products.
According to some studies, specialty pharmaceuticals now account for 25 percent of healthcare costs and represent a rapidly growing area of spending in healthcare. By 2019 some expect that specialty drugs will represent 50 percent of overall Rx drug spending. Many of the new drugs filed for marketing in the United States by the FDA are specialty pharmaceuticals.
Home administration offers significant savings opportunities.
Market differentiation / competitive positioning
Generics/biosimilars pose major threats to branded pharma. So, to enhance the competitive posture of branded products vs. generics/biosimilars, branded pharma can differentiate products and add value—and revenue—by providing related services using mHealth technologies.
In addition to the benefits for specialty pharma and competitive positioning, mHealth technologies offer opportunities to make more effective a risk evaluation and mitigation (REMS) program to ensure the safety of products, to foster concordance among healthcare stakeholders, and to help improve Pharma's overall public image.
Pharma often partners with device manufacturers, which are now also driven to embrace a service model. When the patent owner/licensor sells products, services or systems practicing the inventions in the portfolio to support your pharma products their stakeholder partners also benefit.
For Patients
For patients, benefits can include:
- being able to remain active and mobile
- having tools wherever they go that help them take actions required to make/keep themselves well
- opportunities for initial, and refresher training as well as immediate intervention as necessary
- fewer problems related to polypharmacy, an increasing problem, especially among the aging population
- enhanced potential for emergency response if and when required
- lower overall healthcare cost by avoiding face-to-face healthcare facility or doctor visits, co-pays and other out-of-pocket expenses
- fewer missed workdays
- reduced dependency on caregivers
For Medical Services
For medical services providers, benefits can include:
- better control of medications through monitoring before, during and after administration with two-way communication between stakeholders (e.g., patient and caregiver)
- opportunities for initial, and refresher training as well as immediate intervention as necessary
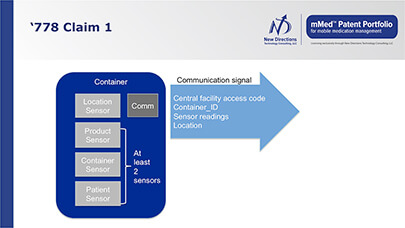
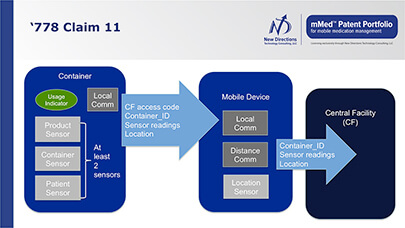
- being able to integrate medication dosing information with other vital signs, medical records, diagnostic information, environmental conditions and geo-location
- avoiding unnecessary face-to-face patient interactions
- greater potential for meaningful medication therapy management
- greater ability to secure reimbursement for remote medication therapy management
- creating stronger bonds with patients
- being able to prioritize patients who need more care
- real-time and sensor-verified medication adherence information
- availability of data on condition or use of patient medication to assure regimen is followed
- assurance of medication replenishment
- automated recordings of events related to medication and the patient's condition by entering dosing information from a system based on the mMed patent portfolio into the patient's electronic medical record
- fewer hospital readmissions
For Healthcare Payers
For healthcare payers, the benefit is clear:
The use of mobile medication management systems can improve patient outcomes and avoid re-hospitalizations and emergency-room visits. This complements pay-for-performance models, such as accountable care organizations (ACOs) and evolving CMS mHealth reimbursement programs.
Payers — patients, caregivers, employers, insurers, government and taxpayers — are working toward solutions that both improve outcomes and reduce patient use of expensive medical services, including emergency rooms and batteries of tests. Solutions that increase adherence to medication regimens help achieve these goals, resulting in decreased overall costs.
For Digitization of Healthcare
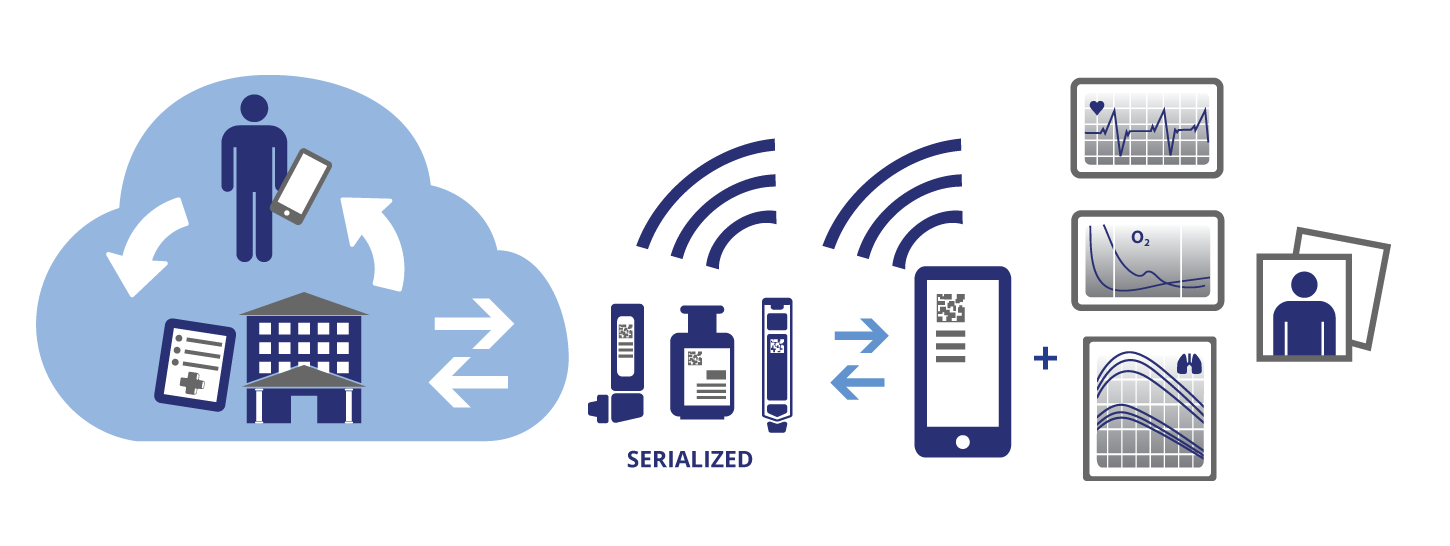
Smart application of automated identity and data capture (AIDC) technology can have broad-reaching advantages to all stakeholders. Manufacturers that track their products to the patient will be able to add value by providing direct-to-patient services, not only helping to improve outcomes, but also gaining real-world data to prove drug benefits to payers.
Legislation and regulations are now in place for the digitization of healthcare.
There also is great potential for enabling the tracking and tracing of medical devices and pharmaceuticals using AIDC, an umbrella term for collecting data electronically. AIDC is widely used in industries other than healthcare.
The mMed patent portfolio has claims that are enabled by 2013 final Unique Device Identifier FDA rulemaking and the Drug Supply Chain and Security Act. Until late 2013, other than a minimally informative, linear National Drug Control barcode requirement for Rx pharmaceutical products, there were no regulations requiring standardized systems for AIDC on healthcare products in the United States.
FDA final rulemaking and legislation in 2013 moved toward defining standards for AIDC for medical devices. Legislation on prescription pharma products followed. These facilitate tracking healthcare products "into the wild" and will lead to better understanding patient outcomes related to the use of those products. For information on serialization see
PDA Letter Napoleon Monroe bylined articles. For more on track and trace, see
Pharmalot article and
FDA.gov.